Why the Netherlands is a Premier Location for Global Clinical Trials and Research
A collaborative approach and culture of knowledge-sharing give life sciences and health companies a competitive edge in the Netherlands
Stepping into the world of clinical trials in the Netherlands means embarking on a journey where science, innovation and patient care converge in the heart of Europe. With an advanced healthcare infrastructure, academic excellence, a robust patient pool and overall ease-of conducting clinical trials, the Netherlands is a top location choice for life sciences and health companies.
The country has earned a prominent position on the global stage for clinical research, as highlighted by the EU Benchmark Clinical Research – the Netherlands as a clinical trial location in Europe – a study commissioned by the Dutch Clinical Research Foundation (DCRF), the Association of Innovative Medicines (VIG), the Invest in Holland Network, Health~Holland and others. The results underscore exceptional performance in clinical research across diverse fields such as oncology, autoimmune diseases, rare diseases and nervous system disorders. It also showcases innovative Dutch leadership and robust infrastructure and logistics.
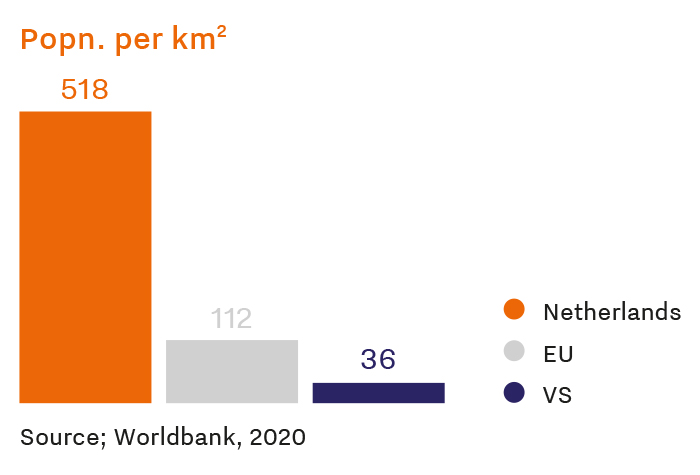
The Netherlands ranks 7th among European countries in absolute number of trial initiations between 2018 and 2022. When adjusted for population within Europe, the country is a top contender for clinical trials worldwide. In fact, the Netherlands initiates close to three times more trials than its European counterparts.
The Netherlands as a hub for clinical trials
The Netherlands is known as Europe’s connected life sciences and health metropolis. This, coupled with the country’s geographical position in the heart of the continent, makes it an ideal location for clinical trials. The Netherlands is easily accessible to a diverse pool of patients and researchers collaborating from across Europe and beyond at state-of-the-art facilities.
As an example, the Dutch Medicines Evaluation Board and the Central Committee on Research Involving Human Subjects are pivotal to ensure clinical trial safety and ethical conduct. These institutions not only uphold rigorous standards and adhere to EU regulations, but they actively partner with sponsors of studies and researchers to smoothen the approval process. In addition, the Netherlands also offers cost-effective advisory services from the European Medicines Agency (EMA), which is headquartered in the country. And, as one of the least expensive countries for comprehensive advice, the approval process for Advanced Therapy Medicinal Products (ATMP) clinical trials is expedited.
To incentivize research within its borders, the Dutch government fosters collaborations between academia and industry, resulting in a synergy of expertise and resources. Companies that locate in the Netherlands can benefit from tax incentives, grants and subsidies.
This support and efficiency makes the country a trusted environment for researchers. Finally, pragmatic and efficient decision-making in the Netherlands advances medical innovation.
Leading the way in clinical research innovation and collaboration
In the Netherlands, clinical research goes beyond scientific exploration. The country boasts the highest concentration of life science and health knowledge institutes globally. In addition, the Netherlands is home to a staggering 65,000 life science and health professionals and 7,800 clinical researchers. There are also eight top-ranking University Medical Centers in the Netherlands. Finally, contract research organizations offer more than 300 lab facilities specialized or suitable for clinical research. This abundance of expertise allows for the efficient conduct of clinical trials.
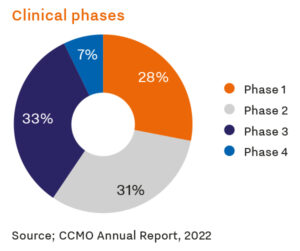
Moreover, the Netherlands provides a conducive environment for novel interventions, assessing and improving efficacy, safety and potential to enhance the quality of life. With approximately 600 new clinical drug trials launched annually and a participant pool of around 60,000, the Netherlands supports therapeutic advancements in diverse specialty areas.
As an example, the Dutch biotech ecosystem is renowned for its excellence in oncology, metabolic and endocrinology clinical trials. Researchers on these trials are seamlessly connected with university medical centers (UMCs), attracting multinational clinical research firms like TASK, which established research facilities in the Netherlands. Proven expertise and the open attitude of UMCs attract clinical research firms.
Positioning innovative companies for success
The Netherlands is at the forefront of clinical trials. The country provides an ideal environment and strategic location for companies seeking to embark on groundbreaking research. Advanced health care infrastructure and streamlined regulatory processes make it a leader in European clinical trials.
Furthermore, top academic institutions and government initiatives create fertile ground for innovation and discovery. This environment cements the Netherlands as the ideal location for companies looking to advance clinical research opportunities. Contact us to learn more.
6 March 2024